Improving scientific knowledge on food contaminants to ensure safer products
Task Force Information
Objectives
As food contaminants poses a threat to consumers’ health, it is crucial to improve our knowledge about them. As such, the Food Contaminants Task Force.
- Advance scientific knowledge on food contaminants – process-related contaminants, natural toxins, and other environmental contaminants – particularly focusing on assessing impacts on human health
- Address research gaps in toxicity, exposure, and analytical aspects of food contaminants
- Review & evaluate mitigation measures and support for risk management strategies for food contaminants.
Task Force Members
| Neil Buck - Chair | General Mills | Corporate Toxicologist | CH |
| Michelangelo Pascale – Co-Chair | Institute of Food Sciences, ISA-CNR | Director | IT |
| Michele Suman – Vice-Chair | Barilla G&R Fratelli | Head of Food Safety & Authenticity | IT |
| Karsten Harms | Südzucker Group | Senior Manager Molecular Biology | DE |
| Clare Hazel | Premier Foods | Group Science Manager | UK |
| Sue O’Hagan | PepsiCo International | Director Scientific Affairs | UK |
| Mónica Molero Monfort | Importaco | Operative Quality Director | ES |
| Gloria Pellegrino | Luigi Lavazza | Scientific Research Manager | IT |
| Dr Daniel Ribera | Cargill | Senior Advisor Regulatory and Scientific Affairs | BE |
| Natalie Thatcher | Mondelēz International | Global Lead for Toxicology | UK |
| Mathilde Bergal | Danone | Food Safety & Toxicology Scientist | FR |
| Keng Ngee Toh | Ajinomoto | Manager, Quality assurance and regulatory science | FR |
| Evangelia Mavromichali | Abbott | Senior Regulatory Affairs Specialist | BE |
| Armando Venâncio* | Center of biological engineering - Univerity of Minho | Associate Professor | PT |
| Konrad Korzeniowski | ILSI Europe | Scientific Project Manager | BE |
* Scientific Advisors
Contact Information
For more detailed information, please contact Konrad Korzeniowski at kkorzeniowski@ilsieurope.be
Activity Overview
Ongoing
- Risk-Benefit Assessment
An expert group on risk-benefits assessment of foods explores the reasons for limited application and seek practical solutions to increase the utility of this methodology in foods.
- Prioritisation of natural toxins for risk management action
The Expert Group establishes a framework to prioritise natural toxins in foods following a risk-based approach. Based on the evidence and scale of risk to consumers, and the potential for risk mitigation, the framework will enable the differentiation between mycotoxins or phytotoxins where risk management action is both warranted and likely to be effective based on available evidence, and highlight potential knowledge gaps.
Start date: Nov 2022 | End date: Nov 2024
- Food contaminant definitions
The Expert Group focuses on existing definitions, their clarity and consistency. This activity un-picks the perceptual underpinning of those different definitions, the key parameters that drive them and the different stakeholders who use them. It identifies the possibility to consolidate a global definition of ‘food contaminant’ and exploresthe value of using the above understanding as a communication tool with food chain stakeholders.
Start date: Feb 2023 | End date: Feb 2024
Upcoming
- Early Career Scientist Seminar
A seminar for Early Career Scientists will take place in Q1 2025. Find more info here.
Expert Groups
Update on Risk-Benefit Assessment of foods: approaches to facilitate application
Background and Objectives
All foods contain chemical and biological impurities some of which may be viewed as contaminants of toxicological or microbiological relevance, depending on the definition used. Classical risk assessment does not reconcile the benefit to health of the food against the potential effects of the contaminants or the impact of mitigation measures that may be applied to reduce contaminants. A contaminant-centric view of a food can result in an incomplete understanding of the net health benefits of food by groups such as policy makers.
As such, methods for the comparison between benefits and risks have been developed, including the publication of guidance materials. Despite the availability of guidance, there have been a limited number of examples of risk-benefit analysis being used as an input for the risk management of foods by food safety agencies.
Output
This activity will review the evolution and application of risk-benefit assessment since its infancy, and thereby understand:
- Why risk-benefit assessment has not been more widely applied by food safety agencies in Europe?
- What are the available applications of RBA since the publication of guidance and what are the lessons learned?
- Whether existing guidance can be amended to improve applicability?
- The above learnings will be tested via a limited number of worked examples and summarized in a peer-reviewed publication.
Expert Group Members
| Géraldine Boué - Chair | ONIRIS | Senior Lecturer in Food Safety | FR |
| Neil Buck - Vice-Chair | General Mills | Corporate Toxicologist | CH |
| Ricardo Assunçao | IUEM - Instituto Universitário Egas Moniz | Assistant Professor | PT |
| Nils Billecke | Cargill | Senior Nutrition Scientist | BE |
| Alan Boobis | Imperial College London | Emeritus Professor of Toxicology | UK |
| Ana Catarina Carvalho | University of Porto | PhD Student | PT |
| Michele Suman | Barilla G&R Fratell | Head of Food Safety & Authenticity | IT |
| Natalie Thatcher | Mondelēz International | Global Lead for Toxicology | UK |
| Gloria Pellegrino | Luigi Lavazza | Scientific Research Manager | IT |
| Lea Sletting Jakobsen | Technical University of Denmark | Senior Researcher | DK |
| Sue O'Hagan | PepsiCo | Director of Scientific Affairs | UK |
| Hans Verhagen | Food Safety and Nutrition Consultancy | Scientific Regulatory Expert | NL |
| Jossie Garthoff | Danone | Global Food Safety Scientific Affairs Leader | NL |
| Konrad Korzeniowski | ILSI Europe | Scientific Project Manager | BE |
Approaches to Facilitiate Application Prioritisation of Natural Toxins for Risk Management
| Background and objectives
In its simplest form, risk is the product of hazard (i.e., toxic potency of a chemical) and exposure (or dose). ‘’Hazard-based’’ decision-making is based solely on hazard without any consideration of exposure. The development of mitigation strategies should prioritize mycotoxins that regularly occur at undesirable levels in commonly consumed commodities, wherein both the toxicological profiles and effectiveness of mitigation are understood with a reasonable degree of certainty. The ultimate goal of mycotoxin mitigation is to prevent adverse health effects caused by foodborne exposure to mycotoxins while reserving nutritional and organoleptic quality of food. Based on the evidence and scale of risk to consumers, and the potential for risk mitigation, the framework will enable the differentiation between mycotoxins or phytotoxins where risk management action is both warranted and likely to be effective based on available evidence. Through case-studies, this framework will also highlight potential knowledge gaps. Output This activity will establish a framework for the prioritization of natural toxins found in food following a risk-based approach (Decision Tree). Based on the evidence and scale of risk to consumers, and the potential for risk mitigation, the framework will enable the differentiation between mycotoxins or phytotoxins where risk management action is both warranted and likely to be effective based on available evidence. Through case-studies, this framework will also highlight potential knowledge gaps. |
Expert Group Members
| Armando Venancio - Chair | Center of biological engineering - Univerity of Minho | Associate Professor | PT |
| Michele Suman - Vice-Chair | Barilla G&R Fratelli | Head of Food Safety & Authenticity | IT |
| Neil Buck | General Mills | Corporate Toxicologist | CH |
| Monica Molero | Importaco | Operative Quality Director | ES |
| Clare Hazel | Premier Foods | Group Science Manager | UK |
| Doris Marko | Universität Wien | Researcher | AT |
| Angela Mally | University of Wuerzburg | Chair of Toxicology | DE |
| Elisabeth Varga | University of Vienna | Senior Scientist | AT |
| Mohamed Fathi Abdallah | Ugent | Post Doctoral Researcher | BE |
| Maxence Oboeuf | Danone | Food Safety and Toxicology Scientist | NL |
| Gerrit Spijers | General Health Effects Toxicology Safety Food | Expert in Food Safety | FR |
| Angel Medina Vaya | Cranfield University | Professor | UK |
| Dr Konrad Korzeniowski | ILSI Europe | Scientific Project Manager | BE |
Food Contaminants Definitions
Background and Objectives
Chemical contaminants are a major focus of risk assessment and management, however there is no common definition of what constitutes a ‘food contaminant’. It is not clear which substances fall into scope of being considered a ‘food contaminant’, and it is not clear what criteria define a substance to be a ‘food contaminant’. As such there is confusion between stakeholders when there is any communication on the subject of ‘food contaminant’. As communication between stakeholders about food contaminants is frequent, there is a pressing need to explore understanding amongst stakeholders and seek clarity on how the term should be defined and therefore used. Activity will un pick the perceptual underpinning of those different definitions, the key parameters that drive them and the different stakeholders that use them. Moreover, identify if it is possible to consolidate a global definition of ‘food contaminant’ and explore the value of using the above understanding as a communication tool with food chain stakeholders.
Output
Overall, the research would provide a comprehensive understanding of the diverse definitions of ‘food contaminant’ among stakeholders, examine the factors influencing these definitions, evaluate the possibility of achieving a global definition, and highlight the potential benefits of using this understanding as a communication tool with stakeholders in the food chain.
Expert Group Members
Publications
Newest to Oldest
The role of endogenous versus exogenous sources in the exposome of putative genotoxins and consequences for risk assessment
Archives of Toxicology, 2022
Commissioned by the Food Contaminants Task Force.
Mineral oil risk assessment: Knowledge gaps and roadmap. Outcome of a multi-stakeholders workshop
Trends in Food Science & Technology, 2021
Commissioned by the Process-Related Compounds & Natural Toxins and the Packaging Materials Task Forces.
Practical Guidance to Mitigation of Mycotoxins During Food Processing
ILSI Europe Report Series, 2019
Commissioned by the Food Contaminants Task Force.
Exposure Assessment of Process-Related Contaminants in Food by Biomarker Monitoring
Archives of Toxicology, 2018
Commissioned by the Food Contaminants Task Force.
Impact of Food Processing and Detoxification Treatments on Mycotoxin Contamination
Mycotoxin Research, 2016
Commissioned by the Food Contaminants Task Force.
- the lack of validated and standardized analytical methods for relevant food matrices, and
- gaps in assessing the risk for consumers' health.
The consensus is that the lack of standardized, validated analytical methods able to assure good inter-laboratory reproducibility is the main gap underlining most of the existing difficulties to understand MOH.
In order to conduct adequate substance identification and quantification for input into risk assessment, the need for confirmatory methods that provide a detailed characterization of the unresolved complex mixtures needs to be solved.
The limited number of surveys covering a wide range of foods and enough samples to detect major sources of contamination other than packaging in paperboard also hinders reliable exposure estimation.
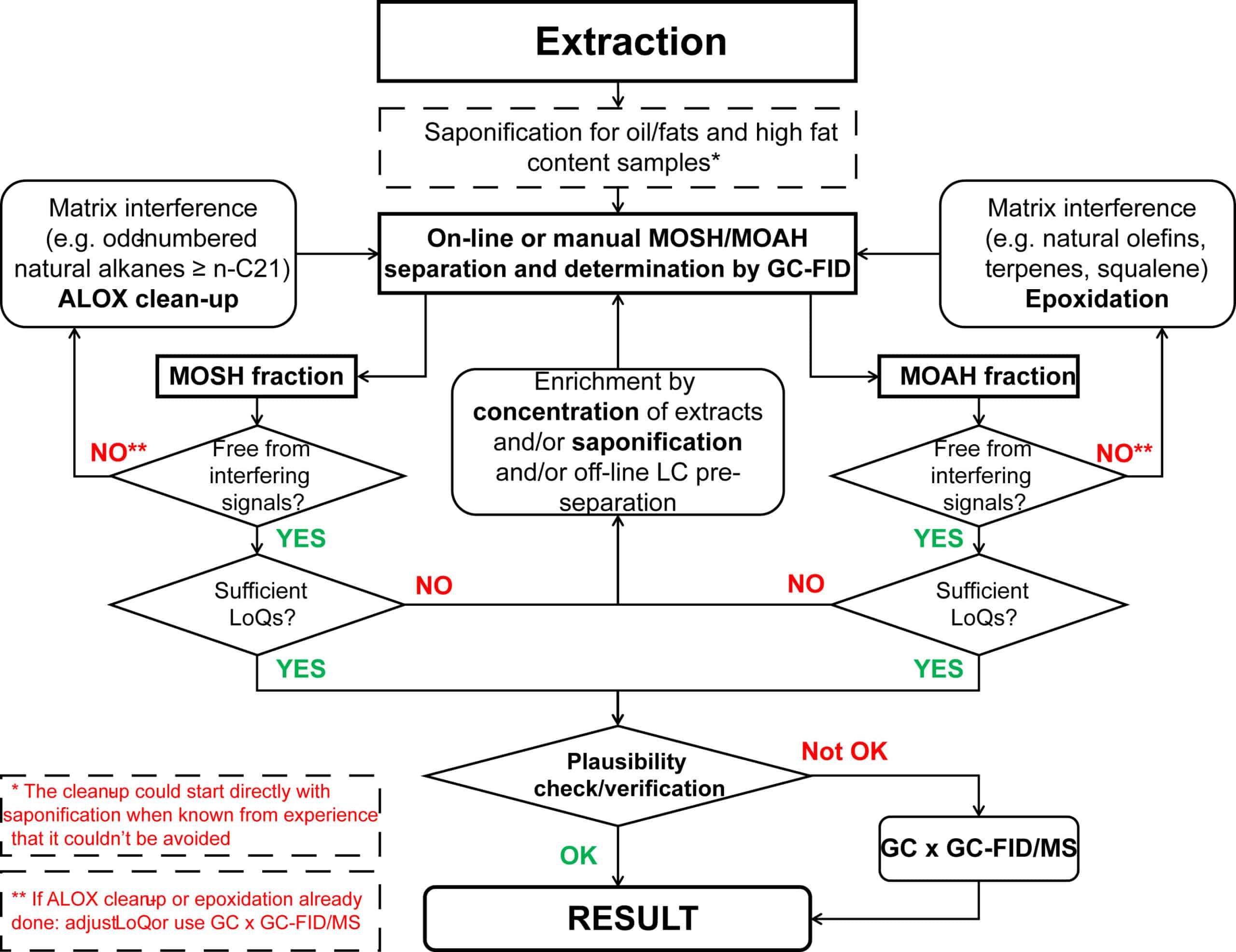
Decision tree to identify auxilary methods. (Adapted from Bratinova & Hoekstra, 2019)
Industry sectors represented in the workshop
- Food & Drink
- Mineral Oil/Waxes
- Testing Laboratories
- Analytical Instruments
- Food Contact Materials
- Cosmetics
- Petroleum
Read the full-text article here
Scientific abstract Expand BackgroundIn recent years there have been significant advancements in the understanding of mineral oil hydrocarbons (MOH) in foods and their potential risk to health. However, important gaps in knowledge remain, such as the lack of validated and standardized analytical methods for relevant food matrices and gaps in assessing the risk for consumers' health. Scope & approach
A workshop was organized by the European Branch of the International Life Science Institute to identify knowledge gaps in analytical methods, assessment of exposure, hazard characterisation, and risk assessment of MOH. This work captures the outcome of the workshop and builds upon it by combining the perspectives of the participants with an updated review of the literature to provide a roadmap for future management of the topic. Key findings and conclusions
Most participants to the workshop agreed that the key issue underlying many of the knowledge gaps in the field of MOH risk analysis and management is the lack of standardized, validated analytical methods able to assure good inter-laboratory reproducibility and to enable understanding of MOH occurrence in foods. It has been demonstrated that method EN 16995 used for MOH determination in vegetable oils and fats is not reliable below 10 mg/kg of food. There is also a need for confirmatory methods that provide a detailed characterization of the unresolved complex mixture observed from one-dimensional chromatographic methods. This is required to enable adequate substance identification and quantification for input into risk assessment. A major gap in the exposure estimation is the limited number of surveys covering a wide range of foods and enough samples to detect major sources of contamination other than packaging in paperboard. Data on concentration of MOH fractions in human body needed to determine internal exposure estimates is scarce. Data relating concentration in tissues with personal data, lifestyle, food intake and the use of cosmetics are needed to clarify the complex system of distribution of MOSH in the body and to possibly establish relationship between external and internal exposure. Additional toxicological studies to better characterize the hazards of relevant MOH are required for a better human health risk assessment. Keywords Expand
Mineral oil hydrocarbon, Risk assessment, Exposure assessment, Food contaminant, MOSH, MOAH
Number of participants in the workshop 61 from Academica, Public organisations, and Industry. EN 16995 used for MOH determination in vegetable oils and fats is not reliable below 10 mg/kg of food. Main indetified gaps in the knowledge of Mineral Oil Hydrocarbons 8To enable human risk assessment, the performance of toxicological studies on the relevant MOH mixtures and possibly their components is required.
This work was conducted in collaboration with the Packaging Materials Task Force.
[post_title] => Mineral oil risk assessment: Knowledge gaps and roadmap. Outcome of a multi-stakeholders workshop [post_excerpt] => [post_status] => publish [comment_status] => closed [ping_status] => closed [post_password] => [post_name] => mineral-oil-risk-assessment-knowledge-gaps-and-roadmap-outcome-of-a-multi-stakeholders-workshop [to_ping] => [pinged] => [post_modified] => 2023-01-04 16:23:23 [post_modified_gmt] => 2023-01-04 16:23:23 [post_content_filtered] => [post_parent] => 0 [guid] => https://ilsi.eu/?post_type=publication&p=9231 [menu_order] => 0 [post_type] => publication [post_mime_type] => [comment_count] => 0 [filter] => raw ) [2] => WP_Post Object ( [ID] => 6672 [post_author] => 24 [post_date] => 2019-09-05 10:10:48 [post_date_gmt] => 2019-09-05 10:10:48 [post_content] => [post_title] => Practical Guidance to Mitigation of Mycotoxins During Food Processing [post_excerpt] => [post_status] => publish [comment_status] => closed [ping_status] => closed [post_password] => [post_name] => practical-guidance-to-mitigation-of-mycotoxins-during-food-processing [to_ping] => [pinged] => [post_modified] => 2023-01-04 16:28:50 [post_modified_gmt] => 2023-01-04 16:28:50 [post_content_filtered] => [post_parent] => 0 [guid] => https://ilsi.eu/?post_type=publication&p=6672 [menu_order] => 0 [post_type] => publication [post_mime_type] => [comment_count] => 0 [filter] => raw ) [3] => WP_Post Object ( [ID] => 5280 [post_author] => 24 [post_date] => 2018-02-22 08:19:34 [post_date_gmt] => 2018-02-22 08:19:34 [post_content] => [post_title] => Exposure Assessment of Process-Related Contaminants in Food by Biomarker Monitoring [post_excerpt] => [post_status] => publish [comment_status] => closed [ping_status] => closed [post_password] => [post_name] => exposure-assessment-of-process-related-contaminants-in-food-by-biomarker-monitoring [to_ping] => [pinged] => [post_modified] => 2023-01-04 16:31:34 [post_modified_gmt] => 2023-01-04 16:31:34 [post_content_filtered] => [post_parent] => 0 [guid] => http://ilsi.eu/?post_type=publication&p=5280 [menu_order] => 0 [post_type] => publication [post_mime_type] => [comment_count] => 0 [filter] => raw ) [4] => WP_Post Object ( [ID] => 2907 [post_author] => 24 [post_date] => 2016-09-01 11:45:15 [post_date_gmt] => 2016-09-01 11:45:15 [post_content] => [post_title] => Impact of Food Processing and Detoxification Treatments on Mycotoxin Contamination [post_excerpt] => [post_status] => publish [comment_status] => closed [ping_status] => closed [post_password] => [post_name] => impact-of-food-processing-and-detoxification-treatments-on-mycotoxin-contamination [to_ping] => [pinged] => [post_modified] => 2023-01-04 16:27:10 [post_modified_gmt] => 2023-01-04 16:27:10 [post_content_filtered] => [post_parent] => 0 [guid] => http://ilsi.eu/?post_type=publication&p=2907 [menu_order] => 0 [post_type] => publication [post_mime_type] => [comment_count] => 0 [filter] => raw ) ) [post_count] => 5 [current_post] => -1 [before_loop] => [in_the_loop] => [post] => WP_Post Object ( [ID] => 10609 [post_author] => 24 [post_date] => 2022-03-29 08:35:38 [post_date_gmt] => 2022-03-29 08:35:38 [post_content] =>The "totality" of the human exposure is conceived to encompass life-associated endogenous and exogenous aggregate exposures. Process-related contaminants (PRCs) are not only formed in foods by heat processing, but also occur endogenously in the organism as physiological components of energy metabolism, potentially also generated by the human microbiome. To arrive at a comprehensive risk assessment, it is necessary to understand the contribution of in vivo background occurrence as compared to the ingestion from exogenous sources. Hence, this review provides an overview of the knowledge on the contribution of endogenous exposure to the overall exposure to putative genotoxic food contaminants, namely ethanol, acetaldehyde, formaldehyde, acrylamide, acrolein, α,β-unsaturated alkenals, glycation compounds, N-nitroso compounds, ethylene oxide, furans, 2- and 3-MCPD, and glycidyl esters. The evidence discussed herein allows to conclude that endogenous formation of some contaminants appears to contribute substantially to the exposome. This is of critical importance for risk assessment in the cases where endogenous exposure is suspected to outweigh the exogenous one (e.g. formaldehyde and acrolein).
Link to download the full-text
Keywords ExpandEndogenous exposure; Exposome; Genotoxins; Process-related contaminants
Commissioned by the Food Contaminants Task Force.
[post_title] => The role of endogenous versus exogenous sources in the exposome of putative genotoxins and consequences for risk assessment [post_excerpt] => [post_status] => publish [comment_status] => closed [ping_status] => closed [post_password] => [post_name] => the-role-of-endogenous-versus-exogenous-sources-in-the-exposome-of-putative-genotoxins-and-consequences-for-risk-assessment [to_ping] => [pinged] => [post_modified] => 2023-01-04 16:20:28 [post_modified_gmt] => 2023-01-04 16:20:28 [post_content_filtered] => [post_parent] => 0 [guid] => https://ilsi.eu/?post_type=publication&p=10609 [menu_order] => 0 [post_type] => publication [post_mime_type] => [comment_count] => 0 [filter] => raw ) [comment_count] => 0 [current_comment] => -1 [found_posts] => 13 [max_num_pages] => 3 [max_num_comment_pages] => 0 [is_single] => [is_preview] => [is_page] => [is_archive] => 1 [is_date] => [is_year] => [is_month] => [is_day] => [is_time] => [is_author] => [is_category] => [is_tag] => [is_tax] => 1 [is_search] => [is_feed] => [is_comment_feed] => [is_trackback] => [is_home] => [is_privacy_policy] => [is_404] => [is_embed] => [is_paged] => [is_admin] => [is_attachment] => [is_singular] => [is_robots] => [is_favicon] => [is_posts_page] => [is_post_type_archive] => [query_vars_hash:WP_Query:private] => 2eaaaf21cc48e2e57d27fd54237a0070 [query_vars_changed:WP_Query:private] => [thumbnails_cached] => [allow_query_attachment_by_filename:protected] => [stopwords:WP_Query:private] => [compat_fields:WP_Query:private] => Array ( [0] => query_vars_hash [1] => query_vars_changed ) [compat_methods:WP_Query:private] => Array ( [0] => init_query_flags [1] => parse_tax_query ) )
