Scientifically addressing the main challenges in the safety and quality of food contact materials
Task Force Information
Objectives
For more than 30 years, the Task Force has strived to understand the challenges to ensure safe food contact materials for food consumption by:
- Evaluating food contact materials safety and their interactions with food to ensure consumers’ safety at minimal environmental impact.
- Addressing recent improvements in food production and distribution, leading to an increased sophistication of food packaging.
Task Force Members
| Thomas Gude | ETH Zurich | Lecturer in Food Safety and Analysis | CH |
| Christina Nerin* | University of Zaragoza | Full Professor | ES |
| Christian Kirchnawy* | Austrian Research Institute for Chemistry and Technology | Team Leader | AT |
| Neil Buck | General Mills | Corporate Toxicologist | CH |
| Sigrid Gerold | Mayr-Melnhof Karton | Food Contact Specialist | AT |
| Sami Hamdi | Mondelēz International | Senior Associate Principal Scientist | UK |
| Charlène Lacourt - Chair | Danone Nutricia Research | Toxicologist-Risk Assessor | FR |
| Bastian Knaup | Tetra Pak | Manager Advanced Analytics and Chemical Safety | DE |
| Peter OIdring | Sherwin Williams | Regulatory Affairs Manager | UK |
| Susanne Kunda | Südzucker Group | Manager Product Safety | DE |
| Laurence Gijs | Dow Europe | EHS&S Regulatory Compliance Manager | DE |
| Tina Richter | Swiss Quality testing Services | Laboratory Manager Food Contact Materials | CH |
| Si Wang | PepsiCo International | Senior Scientist in Scientific Affairs | UK |
| Konrad Korzeniowski | ILSI Europe | Scientific Project Manager | BE |
* Scientific Advisors
Contact Information
For more detailed information, please contact Konrad Korzeniowski at kkorzeniowski@ilsieurope.be
Activity Overview
In the pipeline
- Risk assessment and management of potential migrants
An Expert Group will review existing approaches for risk assessment and management of Food Contac Materials (IAS and NIAS) and identify similarities and differences between materials categories (e.g. plastics vs paper or metals) and geographic areas (e.g. EU, US, China, etc.) before recommending the most appropriate approaches to use, particularly for non harmonised food contact materials.
- Sustainable packaging
More information to come soon...
Expert Groups
Coming soon...
Publications
A to Z
Guidance in selecting analytical techniques for identification and quantification of non-intentionally added substances (NIAS) in food contact materials (FCMS)
2022
Food Additives and Contaminants: Part A. 2022. Commissioned by the Packaging Materials Task Force.
Guidance on Best Practices on the Risk Assessment of Non Intentionally Added Substances (NIAS) in Food Contact Materials and Articles
ILSI Europe Report Series, 2015
Mineral oil risk assessment: Knowledge gaps and roadmap. Outcome of a multi-stakeholders workshop
Trends in Food Science & Technology, 2021
Commissioned by the Process-Related Compounds & Natural Toxins and the Packaging Materials Task Forces.
Outlook and Challenges of Nanotechnologies for Food Packaging
2016
Packaging Technology and Science. 2016;29(12): 615-648. Commissioned by the Packaging Materials Task Force.
Packaging Materials 1: Polyethylene Terephthalate (PET) for Food Packaging Applications
2000
ILSI Europe Report Series.
- the lack of validated and standardized analytical methods for relevant food matrices, and
- gaps in assessing the risk for consumers' health.
The consensus is that the lack of standardized, validated analytical methods able to assure good inter-laboratory reproducibility is the main gap underlining most of the existing difficulties to understand MOH.
In order to conduct adequate substance identification and quantification for input into risk assessment, the need for confirmatory methods that provide a detailed characterization of the unresolved complex mixtures needs to be solved.
The limited number of surveys covering a wide range of foods and enough samples to detect major sources of contamination other than packaging in paperboard also hinders reliable exposure estimation.
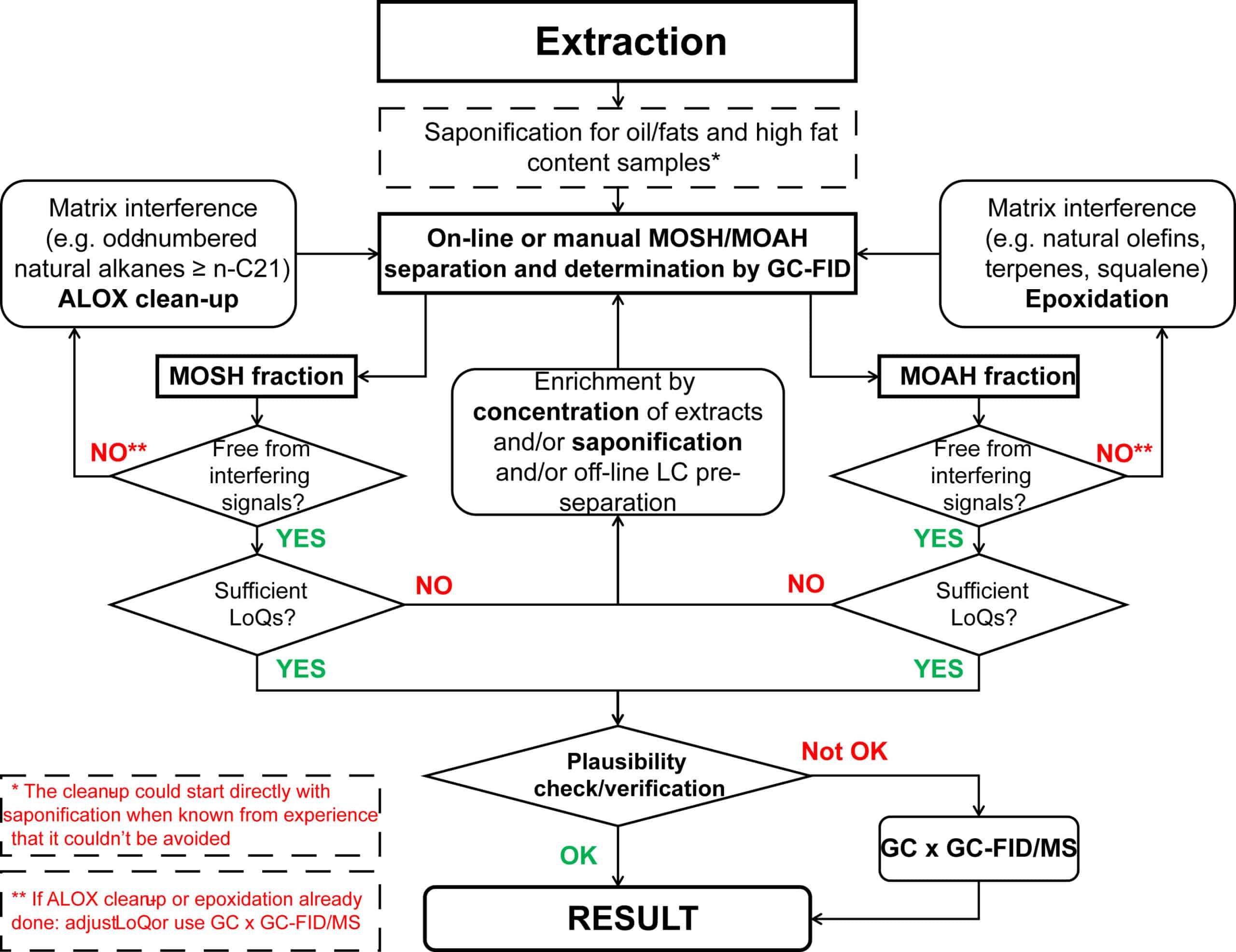
Decision tree to identify auxilary methods. (Adapted from Bratinova & Hoekstra, 2019)
Industry sectors represented in the workshop
- Food & Drink
- Mineral Oil/Waxes
- Testing Laboratories
- Analytical Instruments
- Food Contact Materials
- Cosmetics
- Petroleum
Read the full-text article here
Scientific abstract Expand BackgroundIn recent years there have been significant advancements in the understanding of mineral oil hydrocarbons (MOH) in foods and their potential risk to health. However, important gaps in knowledge remain, such as the lack of validated and standardized analytical methods for relevant food matrices and gaps in assessing the risk for consumers' health. Scope & approach
A workshop was organized by the European Branch of the International Life Science Institute to identify knowledge gaps in analytical methods, assessment of exposure, hazard characterisation, and risk assessment of MOH. This work captures the outcome of the workshop and builds upon it by combining the perspectives of the participants with an updated review of the literature to provide a roadmap for future management of the topic. Key findings and conclusions
Most participants to the workshop agreed that the key issue underlying many of the knowledge gaps in the field of MOH risk analysis and management is the lack of standardized, validated analytical methods able to assure good inter-laboratory reproducibility and to enable understanding of MOH occurrence in foods. It has been demonstrated that method EN 16995 used for MOH determination in vegetable oils and fats is not reliable below 10 mg/kg of food. There is also a need for confirmatory methods that provide a detailed characterization of the unresolved complex mixture observed from one-dimensional chromatographic methods. This is required to enable adequate substance identification and quantification for input into risk assessment. A major gap in the exposure estimation is the limited number of surveys covering a wide range of foods and enough samples to detect major sources of contamination other than packaging in paperboard. Data on concentration of MOH fractions in human body needed to determine internal exposure estimates is scarce. Data relating concentration in tissues with personal data, lifestyle, food intake and the use of cosmetics are needed to clarify the complex system of distribution of MOSH in the body and to possibly establish relationship between external and internal exposure. Additional toxicological studies to better characterize the hazards of relevant MOH are required for a better human health risk assessment. Keywords Expand
Mineral oil hydrocarbon, Risk assessment, Exposure assessment, Food contaminant, MOSH, MOAH
Number of participants in the workshop 61 from Academica, Public organisations, and Industry. EN 16995 used for MOH determination in vegetable oils and fats is not reliable below 10 mg/kg of food. Main indetified gaps in the knowledge of Mineral Oil Hydrocarbons 8To enable human risk assessment, the performance of toxicological studies on the relevant MOH mixtures and possibly their components is required.
This work was conducted in collaboration with the Packaging Materials Task Force.
[post_title] => Mineral oil risk assessment: Knowledge gaps and roadmap. Outcome of a multi-stakeholders workshop [post_excerpt] => [post_status] => publish [comment_status] => closed [ping_status] => closed [post_password] => [post_name] => mineral-oil-risk-assessment-knowledge-gaps-and-roadmap-outcome-of-a-multi-stakeholders-workshop [to_ping] => [pinged] => [post_modified] => 2023-01-04 16:23:23 [post_modified_gmt] => 2023-01-04 16:23:23 [post_content_filtered] => [post_parent] => 0 [guid] => https://ilsi.eu/?post_type=publication&p=9231 [menu_order] => 0 [post_type] => publication [post_mime_type] => [comment_count] => 0 [filter] => raw ) [3] => WP_Post Object ( [ID] => 2677 [post_author] => 24 [post_date] => 2016-07-29 13:45:35 [post_date_gmt] => 2016-07-29 13:45:35 [post_content] => [post_title] => Outlook and Challenges of Nanotechnologies for Food Packaging [post_excerpt] => [post_status] => publish [comment_status] => closed [ping_status] => closed [post_password] => [post_name] => outlook-and-challenges-of-nanotechnologies-for-food-packaging-2016 [to_ping] => [pinged] => [post_modified] => 2017-09-26 08:15:01 [post_modified_gmt] => 2017-09-26 08:15:01 [post_content_filtered] => [post_parent] => 0 [guid] => http://ilsi.eu/?post_type=publication&p=2677 [menu_order] => 0 [post_type] => publication [post_mime_type] => [comment_count] => 0 [filter] => raw ) [4] => WP_Post Object ( [ID] => 2041 [post_author] => 12 [post_date] => 2016-06-28 03:02:12 [post_date_gmt] => 2016-06-28 03:02:12 [post_content] => [post_title] => Packaging Materials 1: Polyethylene Terephthalate (PET) for Food Packaging Applications [post_excerpt] => [post_status] => publish [comment_status] => closed [ping_status] => closed [post_password] => [post_name] => packaging-materials-1-polyethylene-terephthalate-pet-for-food-packaging-applications [to_ping] => [pinged] => [post_modified] => 2016-06-28 03:02:12 [post_modified_gmt] => 2016-06-28 03:02:12 [post_content_filtered] => [post_parent] => 0 [guid] => http://ilsi.eu/?post_type=publication&p=2041 [menu_order] => 0 [post_type] => publication [post_mime_type] => [comment_count] => 0 [filter] => raw ) ) [post_count] => 5 [current_post] => -1 [before_loop] => [in_the_loop] => [post] => WP_Post Object ( [ID] => 10611 [post_author] => 24 [post_date] => 2022-03-29 12:31:37 [post_date_gmt] => 2022-03-29 12:31:37 [post_content] =>Packaging Materials Task Force
SMART PACKAGING
There are numerous approaches and methodologies for assessing the identity and quantities of non-intentionally added substances (NIAS) in food contact materials (FCMs). They can give different results and it can be difficult to make meaningful comparisons. The initial approach was to attempt to prepare a prescriptive methodology but as this proved impossible; this paper develops guidelines that need to be taken into consideration when assessing NIAS. Different approaches to analysing NIAS in FCMs are reviewed and compared. The approaches for preparing the sample for analysis, recommended procedures for screening, identification, and quantification of NIAS as well as the reporting requirements are outlined. Different analytical equipment and procedures are compared. Limitations of today's capabilities are raised along with some research needs.
Link to download the full-text
Keywords ExpandFood contact materials; NIAS; chromatographic methods; food packaging; migration; non-intentionally added substances; plastics
[post_title] => Guidance in selecting analytical techniques for identification and quantification of non-intentionally added substances (NIAS) in food contact materials (FCMS) [post_excerpt] => [post_status] => publish [comment_status] => closed [ping_status] => closed [post_password] => [post_name] => guidance-in-selecting-analytical-techniques-for-identification-and-quantification-of-non-intentionally-added-substances-nias-in-food-contact-materials-fcms [to_ping] => [pinged] => [post_modified] => 2022-06-15 07:54:30 [post_modified_gmt] => 2022-06-15 07:54:30 [post_content_filtered] => [post_parent] => 0 [guid] => https://ilsi.eu/?post_type=publication&p=10611 [menu_order] => 0 [post_type] => publication [post_mime_type] => [comment_count] => 0 [filter] => raw ) [comment_count] => 0 [current_comment] => -1 [found_posts] => 23 [max_num_pages] => 5 [max_num_comment_pages] => 0 [is_single] => [is_preview] => [is_page] => [is_archive] => 1 [is_date] => [is_year] => [is_month] => [is_day] => [is_time] => [is_author] => [is_category] => [is_tag] => [is_tax] => 1 [is_search] => [is_feed] => [is_comment_feed] => [is_trackback] => [is_home] => [is_privacy_policy] => [is_404] => [is_embed] => [is_paged] => 1 [is_admin] => [is_attachment] => [is_singular] => [is_robots] => [is_favicon] => [is_posts_page] => [is_post_type_archive] => [query_vars_hash:WP_Query:private] => 37068a6b1c43cba592308208b8f577db [query_vars_changed:WP_Query:private] => [thumbnails_cached] => [allow_query_attachment_by_filename:protected] => [stopwords:WP_Query:private] => [compat_fields:WP_Query:private] => Array ( [0] => query_vars_hash [1] => query_vars_changed ) [compat_methods:WP_Query:private] => Array ( [0] => init_query_flags [1] => parse_tax_query ) )

