FOOD RELATED CONTAMINANTS
This guidance contains a set of approaches to evaluate available data on target pathogens to support the use of validation studies.
In order to ensure safety of food, a number of control measures need to be implemented by the industry. Validation studies are used to provide evidence that the implemented measures are actually capable of controlling the identified hazard.
Potential to limit the occurrence of discrepancies in the information.
By utilising this guidance, actors involved can identify product and process factors that are essential when designing a validation study. They can thus, select the criteria for identifying an appropriate target pathogen or surrogate organism for a food product and process validation.
Designed for a wide range of food-production professionals.
The document helps food manufacturers, processors, and food safety professionals to better understand, plan, and perform validation studies. It offers an overview of the choices and key technical elements of a validation plan, the necessary preparations including assembling the validation team and establishing prerequisite programs, and the elements of a validation report.
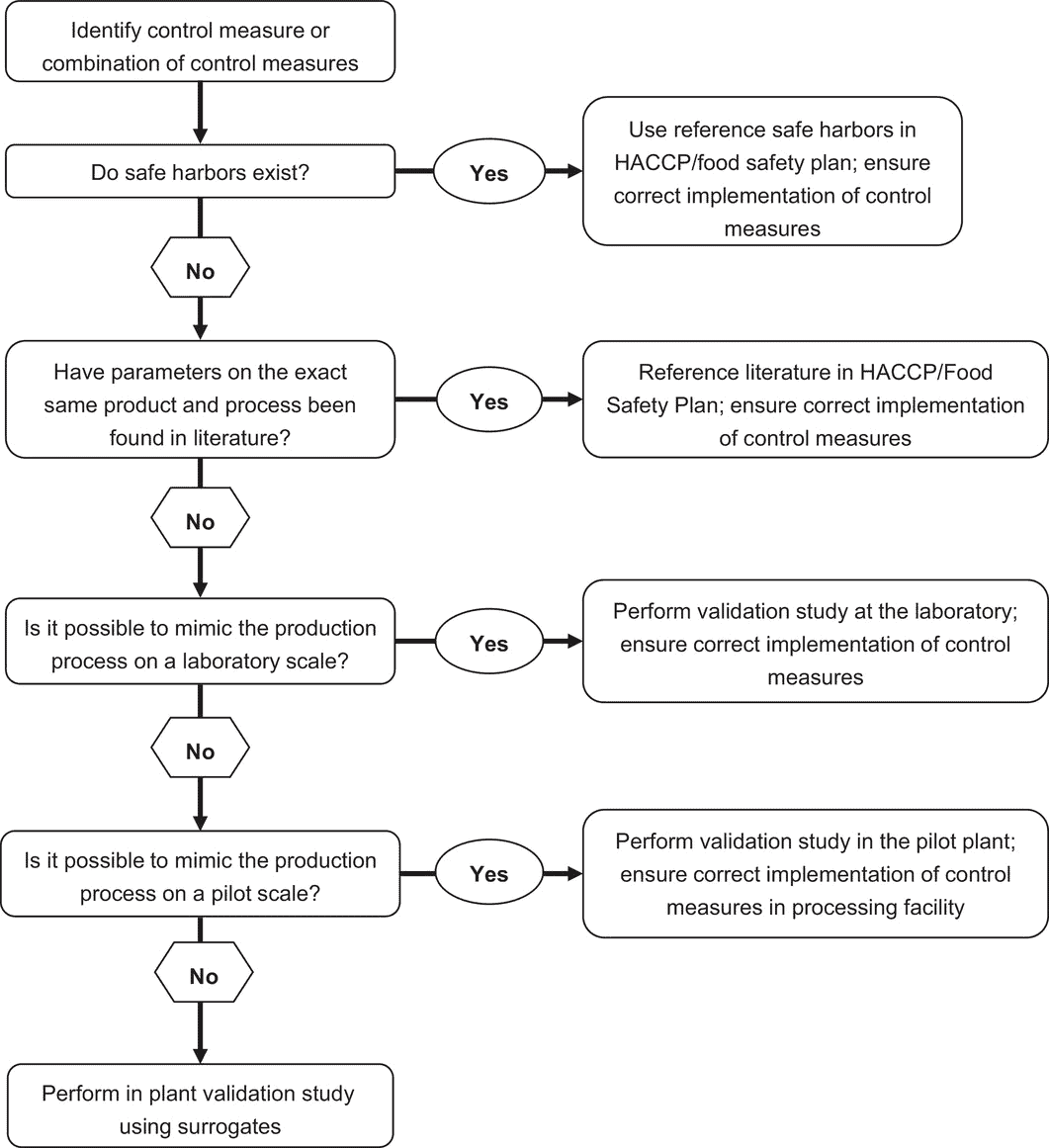
Decision tree to support the decision of when and which validation study approach is most applicable
There is a great interest in the food industry to perform validations in a manner that would be accepted by all parties involved, for example, authorities and customers.
To download this open-access article, please click here.
Validation studies are necessary even when safe harbors are available to ensure correct implementation of control measures.