A European Commission Concerted Action Programme
Supported by the European Commission, DG Research
Thematic Programme 1 – Quality of Life and Management of Living Resources
Key Action 1 – Health, Food and Nutrition
Organised by
International Life Sciences Institute – ILSI Europe
83, Avenue E. Mounier, box 6
B-1200 Brussels
Co-ordinator: Dr. Laura Contor – ILSI Europe
EC-contact: Dr. Jürgen Lucas – Research DG
Duration of the project: from 1 April 2001 to 1 April 2005
Contract number: QLK1-2000-00086
For more information on the PASSCLAIM project, please contact info@ilsieurope.be
Introduction
Objectives
- To produce a generic tool with principles for assessing the scientific support for health-related claims for foods and food components which are eatable or drinkable
- To evaluate critically the existing schemes which assess the scientific substantiation of claims
- To select common criteria for how markers should be identified, validated and used in well-designed studies to explore the links between diet and health
Figure 1: From scientific evidence based on markers for functional foods to types of claims relevant to them
Achievements
- The PASSCLAIM Consensus Document contains consensus criteria on a pan-European level to assess the scientific support for claims on foods, and was widely disseminated among scientists, industry, consumer groups and regulators.
- The PASSCLAIM Consensus Document assists those making and regulating claims.
- PASSCLAIM provides consumers with the assurance that claims are well founded and justified.
- In its comments on Codex Circular Letter 2005/46-FBT on Food Safety Assessment of Food Derived from Recombinant-DNA Plants Modified for Nutritional or Health Benefits, the European Commission referred to PASSCLAIM as follows: “As regards to the methods (…) it is essential that only those recognised by the whole of the international scientific community be used. In the case of the evaluation of health claims, those already being drawn up (PASSCLAIM, Codex) lay down that the quantity and availability of the nutrient about which the claim is made must be verified throughout the lifecycle of the product.”
- EFSA referred to PASSCLAIM in its draft opinion on scientific and technical guidance for preparation and presentation of the application for authorisation of a health claim.
Structure
In order to meet the project objectives, a European network was set up involving academic experts in different aspects of the physiological functions relating to health claims, representatives of public interest groups, regulatory experts and the food industry.
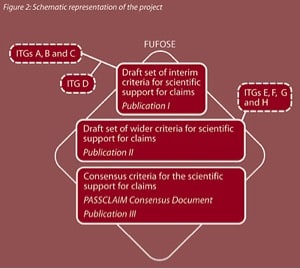
Initially, four “Phase One” Individual Theme Groups (ITGs) were set up. ITGs A, B and C focused on three physiological areas, namely Diet-related cardio-vascular disease (A), Bone health and osteoporosis (B) and Physical performance and fitness (C).
ITG A, B and C held three meetings to critically assess the criteria currently used for the assessment of the scientific support for claims related to their themes. More specifically they aimed to:
- collate potential types of claims (not an exhaustive list but suitable examples) from the perspective of the function related to physiological area, to describe the general principles which would be needed to support these claims and to evaluate the rationale of the scientific support;
- develop a list of criteria that would be used to justify these identified claims: what needs to be done for the development and justification of enhanced function and reduction of disease risk claims and how these claims should be supported in terms of basic science;
- assess the usability of the markers (and their validation) used for the scientific substantiation of the claims.
ITG (D) review of existing processes reviewed the current global situation in terms of schemes to regulate claims and other similar systems and concepts which are universally applied. The report from ITG (D) was made available to ITGs A, B and C while they were drafting their reports. As a consequence ITG A, B and C were aware of the global background as they were making the selection of criteria which are appropriate for their particular physiological theme.
Publication I contains the reports of “Phase One” ITGs (A to D) and a first draft set of interim criteria agreed by the First Plenary Meeting.
The draft set of interim criteria as published in Publication I was used by four new “Phase Two” theme groups, concerned with insulin sensitivity and risk of diabetes (E), diet-related cancer (F), mental state and performance (G) and gut health and immunity (H).
Like the “Phase One” ITGs, the “Phase Two” ITGs elaborated, in key areas for the development of enhanced function or reduction of disease risk claims, a “blueprint” for what needs to be done in terms of scientific research and how claims should be justified. The “Phase Two” theme groups will review and test how the interim criteria can be used in the different physiological areas. They then positioned their criteria with the draft set of interim criteria and produced a draft set of wider new criteria as necessary.
A draft set of wider criteria were then be obtained from the Second Plenary Meeting and were published in Publication II together with the reports of ITGs E to H. This technique of reflective practice and continuous improvement broadened and refined the set of criteria and ensured that many possible methods for the scientific substantiation of claims were considered. This enabled the final PASSCLAIM Consensus Document to contain consensus criteria derived from a very broad basis and ensured its wide applicability.
The Consensus Group then used the outcome of the ITG and Plenary Meetings (Publication I and II) to propose draft consensus criteria for the scientific substantiation of claims. These were agreed by the Third Plenary Meeting and published in the PASSCLAIM Consensus Document, Publication III. The consensus criteria were widely disseminated among the scientific, industrial, regulatory and consumer communities.
Schematic
Management Team
ILSI Europe
Dr Laura Contor: Project coordinator
Dr Loek Pijls: Scientific supervisor
Ms Sandra Tuijtelaars: Project manager
Steering Committee
Chair: Prof. Nils-Georg Asp, SNF – Swedish Nutrition Foundation, SE
Prof. Peter Aggett, University of Central Lancashire, UK
Dr Jean-Michel Antoine, Groupe Danone, FR
Dr France Bellisle, INSERM – Unité 341, FR
Prof. Bruce German, Nestlé, CH
Dr Detlef Müller, Procter & Gamble, DE
Prof. Gerhard Rechkemmer, Technical University of Munich, DE
Dr Hans Verhagen, Unilever Health Institute, NL
Scientific Officer
Dr Jürgen Lucas, European Commission, DG Research, Unit E.2 Health, Food and Environment, BE
Participants
Participants according to their ITG group are listed below:
Applications
- PASSCLAIM offers a practical scientific framework to prepare scientific dossiers supporting claims. This framework ensures that all claims have a firm scientific base. European food manufacturing industry, including SME’s, benefit because of the competitive edge that has been provided.
- PASSCLAIM enables the compilation of guidelines to prepare submissions for claims on foods. This expedites and improves the efficiency of the regulatory review processes.
- Consumers benefit from an improved approach to the scientific support for claims on foods. This integrated strategy generates more consumer confidence in the science base related to claims on foods and better addresses the concerns of consumers.
Publications
PASSCLAIM Consensus Document
Click here to download PASSCLAIM – Consensus on Criteria
PASSCLAIM – Phase One: Preparing the Way
Click here to download PASSCLAIM Overall introduction by Asp and Contor
Click here to download PASSCLAIM ITG A on diet related cardiovascular disease by Mensink et al.
Click here to download PASSCLAIM ITG B on Bone health and osteoporosis by Prentice et al.
Click here to download PASSCLAIM ITG C on Physical Performance and Fitness by Saris et al.
Click here to download PASSCLAIM ITG D on Synthesis and review of Existing Processes by Richardson et al.
Click here to download PASSCLAIM – Report of the Second Plenary Meeting including a set of interim criteria to scientifically substantiate claims on foods by Cummings et al.
Click here to download a poster about PASSCLAIM
Click here to download the PASSCLAIM brochure
PASSCLAIM Phase Two:
Click here to download PASSCLAIM Phase 2 overall introduction by Asp and Contor
Click here to download PASSCLAIM ITG E on Body weight regulation, insulin sensitivity and diabetes risk by Riccardi et al.
Click here to download PASSCLAIM ITG F on Diet-related cancer by Rafter et al.
Click here to download PASSCLAIM ITG G on Mental state and performance by Westenhoefer et al.
Click here to download PASSCLAIM ITG H on Gut health and immunity by Cummings et al.
Click here to download PASSCLAIM – Report of the second plenary
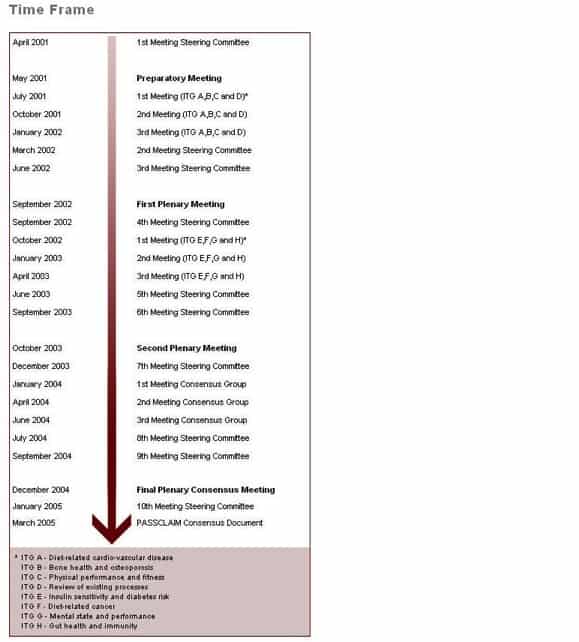
Time Frame
The time frame for the PASSCLAIM project is shown in the schedule below:
Newsflash and Poster
To download the Newsflash, click here
To download the Poster, click here